- Draw the Lewis structure for the polyatomic hydronium (H,o') cation. Be sure to include all resonance structures that satisfy the octet rule.
- Alternate view of lewis dot structure of water: This arrangement of shared electrons between O and H results in the oxygen atom having an octet of electrons, and each H atom having two valence electrons. Multiple bonds can also form between elements when two or three pairs of electrons are shared to produce double or triple bonds, respectively.
Trimethylammonium C3H10N+ CID 3782034 - structure, chemical names, physical and chemical properties, classification, patents, literature, biological activities. Draw Lewis dot structures for the following atoms, ions, or molecules: a. Draw Lewis structures for the following: a. P e.CF b.C iP 28. Draw Lewis structures for the following: a.
Learning Objective
- Apply the rules for drawing Lewis structures to polyatomic ions
Key Points
- Ions are treated almost the same way as a molecule with no charge. However, the number of electrons must be adjusted to account for the net electric charge of the ion.
- When counting electrons, negative ions should have extra electrons placed in their Lewis structures, while positive ions should have fewer electrons than an uncharged molecule.

Term
- polyatomic ionA charged species composed of two or more atoms covalently bonded, or of a metal complex that acts as a single unit in acid-base chemistry or in the formation of salts. Also known as a molecular ion.
The total number of electrons represented in a Lewis structure is equal to the sum of the numbers of valence electrons in each individual atom. Non-valence electrons are not represented in Lewis structures. After the total number of available electrons has been determined, electrons must be placed into the structure.
Lewis structures for polyatomic ions are drawn by the same methods that we have already learned. When counting electrons, negative ions should have extra electrons placed in their Lewis structures; positive ions should have fewer electrons than an uncharged molecule. When the Lewis structure of an ion is written, the entire structure is placed in brackets, and the charge is written as a superscript on the upper right, outside of the brackets. For example, consider the ammonium ion, NH4+, which contains 9 (5 from N and 1 from each of the four H atoms) –1 = 8 electrons. One electron is subtracted because the entire molecule has a +1 charge.
Negative ions follow the same procedure. The chlorite ion, ClO2–, contains 19 (7 from the Cl and 6 from each of the two O atoms) +1 = 20 electrons. One electron is added because the entire molecule has a -1 charge.
Show SourcesBoundless vets and curates high-quality, openly licensed content from around the Internet. This particular resource used the following sources:
http://www.boundless.com/
Boundless Learning
CC BY-SA 3.0.
Phosphate Ion Lewis Structure
http://www.chem1.com/acad/webtext/chembond/cb03.html#SEC2
Steve Lower's Website
CC BY-SA.
http://en.wikipedia.org/wiki/polyatomic%20ion
Wikipedia
CC BY-SA 3.0.
http://en.wikipedia.org/wiki/Lewis_structure
Wikipedia
CC BY-SA 3.0.
https://commons.wikimedia.org/wiki/File:Hypochlorite_Lewis_Structures_V1.svg
Wikimedia
CC BY-SA 4.0.
Nitrate Ion Lewis Structure
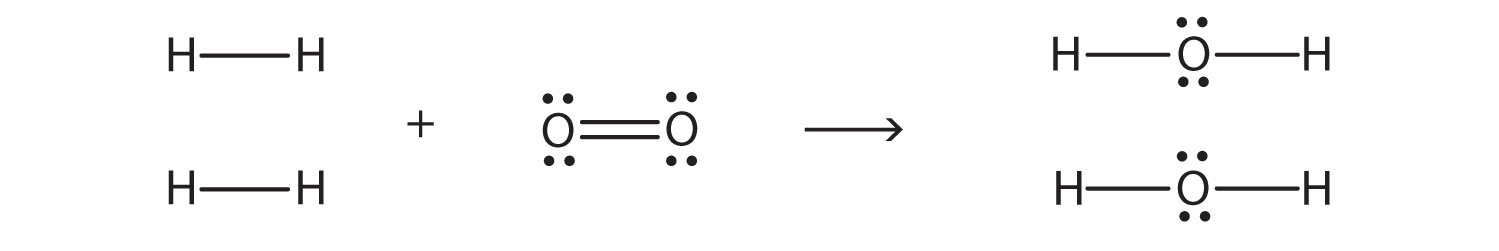

Term
- polyatomic ionA charged species composed of two or more atoms covalently bonded, or of a metal complex that acts as a single unit in acid-base chemistry or in the formation of salts. Also known as a molecular ion.
The total number of electrons represented in a Lewis structure is equal to the sum of the numbers of valence electrons in each individual atom. Non-valence electrons are not represented in Lewis structures. After the total number of available electrons has been determined, electrons must be placed into the structure.
Lewis structures for polyatomic ions are drawn by the same methods that we have already learned. When counting electrons, negative ions should have extra electrons placed in their Lewis structures; positive ions should have fewer electrons than an uncharged molecule. When the Lewis structure of an ion is written, the entire structure is placed in brackets, and the charge is written as a superscript on the upper right, outside of the brackets. For example, consider the ammonium ion, NH4+, which contains 9 (5 from N and 1 from each of the four H atoms) –1 = 8 electrons. One electron is subtracted because the entire molecule has a +1 charge.
Negative ions follow the same procedure. The chlorite ion, ClO2–, contains 19 (7 from the Cl and 6 from each of the two O atoms) +1 = 20 electrons. One electron is added because the entire molecule has a -1 charge.
Show SourcesBoundless vets and curates high-quality, openly licensed content from around the Internet. This particular resource used the following sources:
http://www.boundless.com/
Boundless Learning
CC BY-SA 3.0.
Phosphate Ion Lewis Structure
http://www.chem1.com/acad/webtext/chembond/cb03.html#SEC2
Steve Lower's Website
CC BY-SA.
http://en.wikipedia.org/wiki/polyatomic%20ion
Wikipedia
CC BY-SA 3.0.
http://en.wikipedia.org/wiki/Lewis_structure
Wikipedia
CC BY-SA 3.0.
https://commons.wikimedia.org/wiki/File:Hypochlorite_Lewis_Structures_V1.svg
Wikimedia
CC BY-SA 4.0.
Nitrate Ion Lewis Structure
https://commons.wikimedia.org/wiki/File:Coordinate_Covalent_Bonding.svg
Wikimedia
CC BY-SA 3.0.
